Introduction
Calcium chloride (CaCl₂) is one of the most widely used concrete additives, particularly in cold-weather construction. It accelerates curing, prevents freezing and enhances early strength development. However, improper use can lead to structural risks, including corrosion and durability issues.
This comprehensive guide explores:
- The chemical mechanism behind calcium chloride’s accelerating effects
- Optimal dosage for different temperatures and mix designs
- Critical risks (corrosion, alkali-silica reaction) and mitigation strategies
- Industry standards (ASTM, JGJ/T) for safe application
1. How Calcium Chloride Works: The Science Behind Acceleration
1.1 Hydration Reaction & Temperature Influence
Concrete curing relies on the hydration of cement particles (C₃S, C₂S). Calcium chloride acts as a catalyst, speeding up this process by:
- Increasing ion concentration in the mix promotes faster dissolution of silicates.
- Generating heat through exothermic reactions is crucial in cold weather.
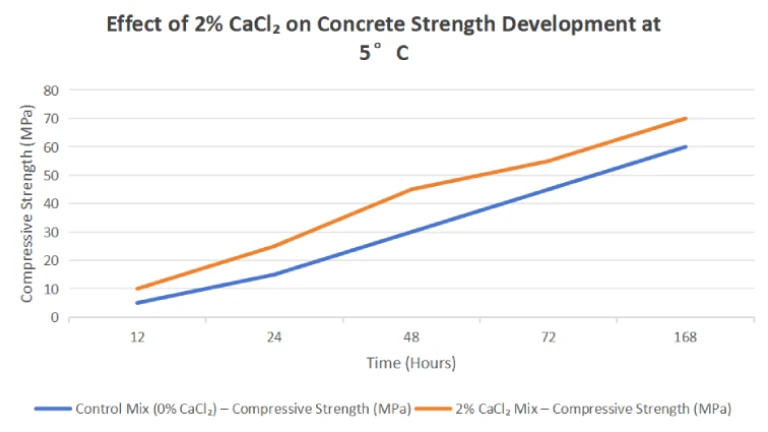
A time-temperature curve (Fig. 1) shows that with 2% CaCl₂, concrete reaches 50% strength 20–30% faster than untreated mixes.
Fig. 1: Strength Development with/without CaCl₂
(Hypothetical data: 2% CaCl₂ reduces setting time by 40% at 5°C.)
1.2 Impact on Microstructure
While CaCl₂ accelerates early strength, excessive use (beyond 2% by cement weight) can:
- Increase porosity due to rapid water consumption.
- Cause shrinkage cracks if drying occurs before full hydration.
2. Engineering Formulations: Dosage, Compatibility & Best Practices
2.1 Recommended Dosage for Cold Weather (-10°C to 5°C)
The optimal CaCl₂ dosage depends on ambient temperature:
| Temperature Range | CaCl₂ (% of Cement Weight) | Expected Strength Gain |
|---|---|---|
| 5°C to 10°C | 1–1.5% | 30–40% at 24 hours |
| -5°C to 5°C | 1.5–2% | 50–60% at 48 hours |
| Below -5°C | 2% + anti-freeze agents (e.g., nitrites) | Prevents freezing, but requires insulation. |
Note: Dosages above 2% are not recommended due to corrosion risks.
2.2 Synergy with Accelerators (Non-Chloride vs. Chloride-Based)
Calcium chloride is often combined with:
- Triethanolamine (TEA): Enhances early strength without increasing corrosion risk.
- Calcium Nitrite: Provides freeze protection while inhibiting rust.
Avoid mixing with:
- Sulfate-based accelerators (e.g., sodium thiocyanate) → Can cause delayed ettringite formation (DEF).
3. Critical Risks & Mitigation Strategies
3.1 Corrosion of Reinforcement Steel
Chlorides depassivate the protective oxide layer on the rebar, leading to electrochemical corrosion.
Prevention methods:
- Epoxy-coated rebar or stainless steel reinforcement.
- Cathodic protection (sacrificial anodes or impressed current).
- Corrosion inhibitors (e.g., calcium nitrite at 10–15% by cement weight).
3.2 Why Prestressed Concrete Bans CaCl₂
Prestressed concrete is highly susceptible to chloride-induced stress corrosion cracking (SCC). ASTM A416 prohibits CaCl₂ in:
- Post-tensioned beams
- Pre-tensioned railway sleepers
3.3 Alkali-Silica Reaction (ASR) Risk
CaCl₂ can exacerbate ASR in reactive aggregates. Mitigation includes:
- Using low-alkali cement (<0.6% Na₂O equivalent).
- Adding pozzolans (fly ash, slag) to reduce permeability.
4. Compliance with Industry Standards
4.1 ASTM D98 vs. JGJ/T 104: Key Differences
| Parameter | ASTM D98 (US) | JGJ/T 104 (China) |
|---|---|---|
| Max Chloride Content | 1% for reinforced concrete | 0.1% for humid environments |
| Testing Method | Potentiometric titration | Volumetric analysis |
| Pre-stressed Ban | Absolute prohibition | Case-by-case evaluation |
Practical Tip: For marine environments, EN 206 (EU) limits chlorides to 0.4% for reinforced concrete.
Conclusion
Calcium chloride remains a cost-effective accelerator for cold-weather concreting, but its risks demand strict adherence to:
✔ Dosage limits (1–2% by weight)
✔ Corrosion prevention (inhibitors, coatings)
✔ Material compatibility (avoid sulfates, prestressed applications)
For critical projects, consider non-chloride alternatives (e.g., calcium formate).

