The Scaling Challenge: Why Industrial Water Softening Is Essential
Industrial water hardness—primarily caused by dissolved calcium (Ca²⁺) and magnesium (Mg²⁺) ions—represents a persistent and costly challenge across industrial water treatment systems. In boilers, heat exchangers, and cooling towers, these ions precipitate under high temperature and pressure, forming hard crystalline scale on metal surfaces.
Even minimal scale accumulation has measurable consequences. Studies show that a scale layer as thin as 0.8 mm (1/32 inch) can reduce heat-transfer efficiency by up to 7%, significantly increasing fuel consumption and operating costs. Left unchecked, scaling accelerates corrosion, shortens equipment lifespan, and raises unplanned downtime risks—making effective Industrial Water Treatment essential for long-term operational stability.
Sodium carbonate (Na₂CO₃)—commonly known as soda ash—plays a critical role in industrial water softening by converting soluble hardness ions into insoluble precipitates. This chemical transformation protects core infrastructure while improving overall energy efficiency.

The Chemical Mechanism: Precipitation-Based Hardness Removal
Sodium carbonate is especially effective in removing permanent hardness, such as calcium sulfate (CaSO₄) and calcium chloride (CaCl₂), which cannot be eliminated through heating alone.
When Na₂CO₃ is added to hard water, a double displacement (precipitation) reaction occurs:
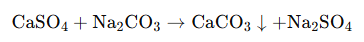
The reaction produces calcium carbonate (CaCO₃)—a compound with extremely low solubility. The solid precipitate can then be efficiently removed via sedimentation, clarification, or filtration before the water enters sensitive downstream equipment.
This mechanism makes sodium carbonate a cornerstone chemical in industrial scale control and hardness management.
Industrial Applications: Sector-Specific Benefits
Sodium carbonate is not a generic additive; its effectiveness depends on precise application within different industrial environments.
Power Generation and High-Pressure Boilers
In power plants, boiler feedwater quality is non-negotiable. Sodium carbonate is widely used as a primary softening agent to reduce calcium levels before demineralization. This prevents scale-related “tube burnout,” a failure mode caused by localized overheating beneath scale layers.
By lowering hardness from approximately 300 ppm to below 50 ppm, facilities can reduce the chemical load on ion-exchange resins by nearly 40%, extending resin life and lowering regeneration costs.
Mining and Hydrometallurgy
Mining operations frequently rely on highly mineralized groundwater. Sodium carbonate improves process water quality for flotation and leaching by preventing calcium ions from interfering with reagents. This directly improves ore recovery rates.
Additionally, soda ash enables closed-loop water reuse systems, allowing up to 80% process water recycling—a critical advantage in arid and water-stressed regions.
Petrochemical and Oil Refining Operations
In oilfields, sodium carbonate is integral to produced water treatment. By removing scale-forming ions before reinjection, it protects downhole equipment and prevents reservoir plugging. Without effective softening, extraction efficiency can decline by 15–20% due to formation damage.
Textile and Paper Manufacturing
In textile dyeing, trace hardness can cause uneven coloration and poor dye fixation. Sodium carbonate simultaneously softens water and buffers pH, ensuring consistent dye performance.
In paper mills, soda ash prevents scale from clogging the fine mesh of Fourdrinier machines, supporting uniform fiber bonding, sheet formation, and thickness control.
Core Advantages of Sodium Carbonate in Water Softening
TThe widespread adoption of Sodium Carbonate (Soda Ash) in industrial water softening is driven by its strong precipitation efficiency, stable alkalinity contribution, and excellent compatibility with large-scale treatment systems.
Cost Efficiency
Soda ash offers one of the lowest cost-per-unit hardness removed, particularly in large-volume systems, outperforming many liquid softening agents.
pH Control and Corrosion Protection
Sodium carbonate provides alkalinity buffering, typically maintaining system pH between 9.5 and 10.5—an optimal range for minimizing acidic corrosion in carbon steel piping.
Process Compatibility
Na₂CO₃ integrates seamlessly with lime-soda softening processes and serves as an effective pretreatment for reverse osmosis (RO) systems. By reducing scaling potential, it can extend RO membrane life by up to 2.5×.
Operational Optimization and Precision Dosing
Modern industrial facilities increasingly rely on hot-process softening to maximize soda ash efficiency. Heating water to approximately 100 °C (212 °F) accelerates precipitation reactions and produces denser sludge that settles more rapidly.
Advanced automation—such as real-time hardness monitoring, turbidity sensors, and automated titration—allows precise dosing control. These technologies can reduce chemical consumption by up to 15%, ensuring optimal performance despite fluctuations in raw water quality.
Sodium Carbonate in the Future of Industrial Water Management
As global water scarcity intensifies, sodium carbonate’s role is evolving beyond conventional softening toward water reuse and resource recovery. By enabling gray water recycling, protecting high-value equipment, and supporting Zero Liquid Discharge (ZLD) strategies, soda ash remains a foundational chemical within integrated industrial chemical solutions for sustainable industrial water treatment.
For industries balancing operational efficiency with environmental responsibility, the strategic application of sodium carbonate is not merely a treatment option—it is a long-term asset protection and water management solution.
